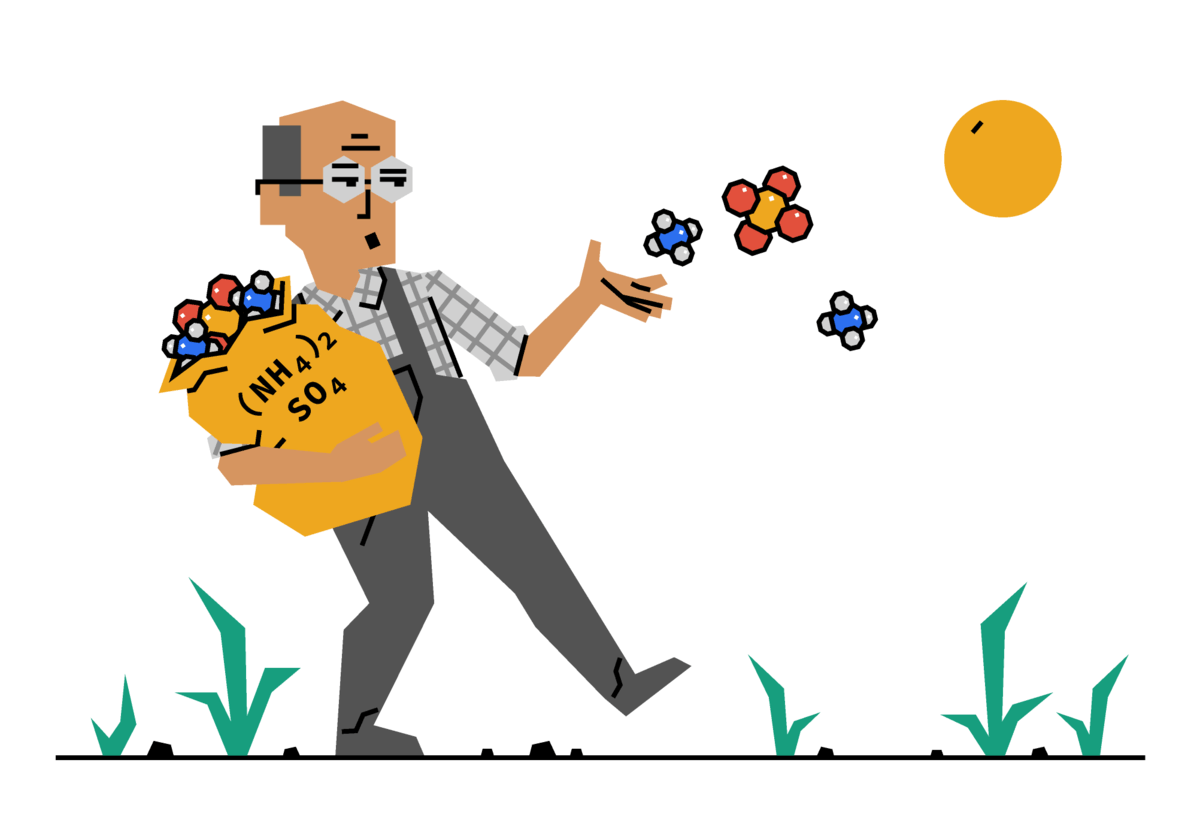
第1600题:ammonium sulfate
A salt called ammonium sulfate, represented by (NH4)2SO4, is a common fertilizer. We produce it in a reaction of ammonia with sulfuric acid, represented by NH3 and H2SO4 , respectively. This reaction can be summarized by a non-balanced equation
NH3 + H2SO4 (NH4)2SO4 .
A molecule of sulfuric acid weighs approximately 5.8 times as much as a molecule of ammonia.
How much sulfuric acid would you need in order to make 100 Kg of ammonium sulfate?
A. About 62 Kg
B. About 74 Kg
C. About 85 Kg
D. About 89 Kg
From brilliant.org